Innovative products for better health
and quality of life

NABOTA®50, 100, 200Units / vial-Inj.
Botulinum toxin type A
Indication
- Temporary improvement in the appearance of moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity in adult patients (Approved in KR, US, EU, Canada)
- Treatment of upper limb spasticity in adult patients (Approved in KR)
- Temporary improvement in the appearance of moderate to severe lateral canthal lines associated with orbicularis oculi activity in adult patients (Approved in KR)
- Treatment of blepharospasm associated with dystonia in adult patients (Approved in KR)
- HI Pure™ Technology (KR patent No.13-39349, US Patent No. 9,512,418)

CareTropin™22.5IU (7.5mg) - Cartridge Pen Type Inj.
Somatropin (human growth hormone)
Indication
- Growth hormone replacement therapy in adults with growth hormone deficiency
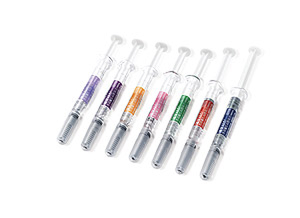
Eposis™2000, 3000, 4000, 5000, 6000, 8000, 10000IU / Pre-filled syringe Inj.
rhEPO (recombinant human erythropoietin)
Indication
- Growth hormone replacement therapy in adults with growth hormone deficiency
Albumin-free

Epodion™2000, 3000, 4000, 10000IU / Pre-filled syringe Inj.
rhEPO (recombinant human erythropoietin)
Indication
- Anemia in chronic renal failure

Easyef™0.5㎎/10㎖ / Topical Solution(Spray)
rhEGF (=recombinant human Epidermal Growth Factor)
Indication
- Diabetic foot ulcer

